views
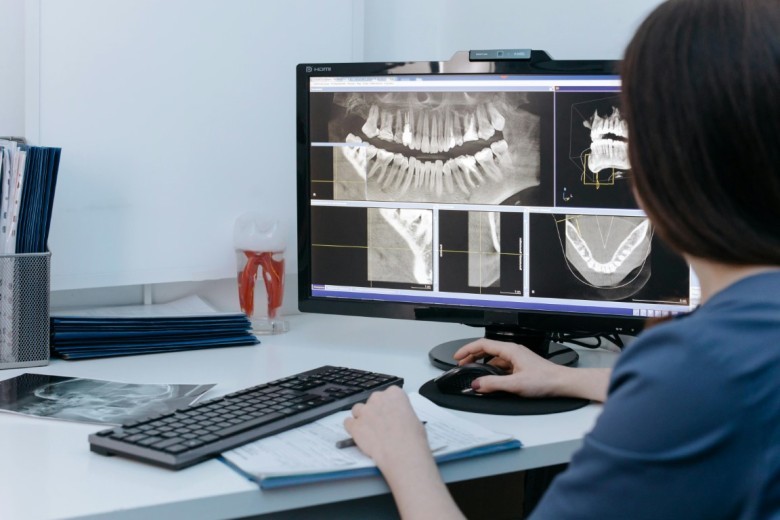
Suboxone, a medication used in the treatment of opioid addiction, has come under scrutiny due to claims of causing severe dental problems. Allegations against the manufacturer, Indivior, suggest that the company failed to adequately warn patients and medical professionals about these potential side effects.
As a result, numerous lawsuits have been filed, seeking compensation for the damages incurred. However, the legal landscape surrounding Suboxone tooth decay claims is complex, requiring a thorough investigation of both evidence and arguments to navigate successfully.
Understanding the Allegations
Allegations against Suboxone are based on claims that the drug's maker, Indivior, did not sufficiently alert the public to the drug's possible dental dangers. Plaintiffs in the Suboxone lawsuit argue that despite knowledge or awareness of these risks, the company prioritized profit over patient safety.
According to the American Journal of Nursing, this assertion is supported by FDA warnings issued in January 2022.
These warnings highlight the dental problems associated with medicines containing buprenorphine, such as Suboxone. Additionally, as per TruLaw, the filing of numerous lawsuits, totaling 358 pending cases as of June 2024, underscores the widespread concern over Suboxone's alleged side effects.
Assessing Medical Evidence
The assessment of medical evidence is crucial in substantiating Suboxone tooth decay claims. Medical experts analyze data from clinical studies and patient reports to establish a causal link between Suboxone use and dental problems.
Notably, DrBicuspid.com highlights that David Sorensen's lawsuit from September 2023 alleges permanent dental damage following Suboxone use.
It highlights the personal testimonies driving these claims. The FDA's requirement for a new warning on Suboxone's prescribing information and patient medication guide in January 2022 reinforces these allegations.
Evaluating Causation
Determining causation is essential in Suboxone tooth decay claims to establish liability. Lawyers and experts meticulously examine the sequence of events leading to the onset of dental issues in Suboxone users.
The Lawsuit Information Center notes that in November 2023, 14 Suboxone lawsuits were filed against Individor in federal courts. The Suboxone teeth decay allegations were consolidated into a multidistrict litigation (MDL) in the Northern District of Ohio in February 2024. This underscores the legal recognition of a potential causal relationship.
Analyzing Regulatory Actions
Past regulatory actions, particularly those by the FDA, provide crucial insights into Suboxone tooth decay claims. The FDA's public announcement in January 2022 regarding dental problems associated with Suboxone reflects the regulatory acknowledgment of these risks.
Furthermore, the FDA's requirement for an updated warning on Suboxone's prescribing information underscores the agency's commitment to informing healthcare providers and patients. The involvement of regulatory authorities adds weight to the allegations of inadequate warning by Indivior.
Considering Defense Strategies
Indivior is likely to employ various defense strategies in response to Suboxone tooth decay lawsuits. The company may challenge the sufficiency of evidence linking Suboxone use to dental problems, citing the complexity of medical causation.
Additionally, legal doctrines such as preemption may be invoked to argue that federal law precludes certain state law claims. The extensive litigation history, including the settlement of an antitrust case for $385 million in October 2023, indicates Indivior's preparedness to contest these claims.
Examining Previous Legal Outcomes and Settlements
Examining previous legal outcomes and settlements provides insight into the potential direction and impact of Suboxone tooth decay claims. Earlier, in 2019 and 2020, the Federal Trade Commission (FTC) reached settlements totaling $60 million in an antitrust class-action lawsuit related to Suboxone.
These settlements illustrate Indivior's history of resolving legal disputes, albeit in different contexts. They also suggest that substantial financial settlements could be possible in the current wave of dental-related claims.
FAQs
Why is Suboxone legal?
Suboxone is legal because it is an FDA-approved medication for the treatment of opioid addiction. It helps reduce withdrawal symptoms and cravings, making it a vital tool in managing opioid dependence under medical supervision.
Is Suboxone still being prescribed?
Yes, Suboxone is still being prescribed. Despite the lawsuits, it remains a widely used treatment for opioid addiction, helping many individuals manage their recovery and reduce the risk of relapse.
Who owns Suboxone?
Suboxone is owned by Indivior, a pharmaceutical company specializing in addiction treatment medications. Indivior was previously a part of Reckitt Benckiser before becoming an independent company.
In conclusion, the Suboxone tooth decay litigation underscores significant concerns about pharmaceutical responsibility and patient safety. The FDA's warnings and the consolidation of numerous lawsuits into multidistrict litigation highlight the gravity of these claims.
Indivior’s defense strategies, which include challenging the causation evidence, will be rigorously tested. This legal battle emphasizes the critical need for transparent communication of drug side effects and robust regulatory oversight. Ultimately, ensuring patient well-being must remain paramount in the development and marketing of medications.




















Comments
0 comment